Harness the power of the sun to create art & charge your devices!
Monday: Solar Science Station Kit
Electrifying Videos and Resources

Shock and Awe: The Story of Electricity
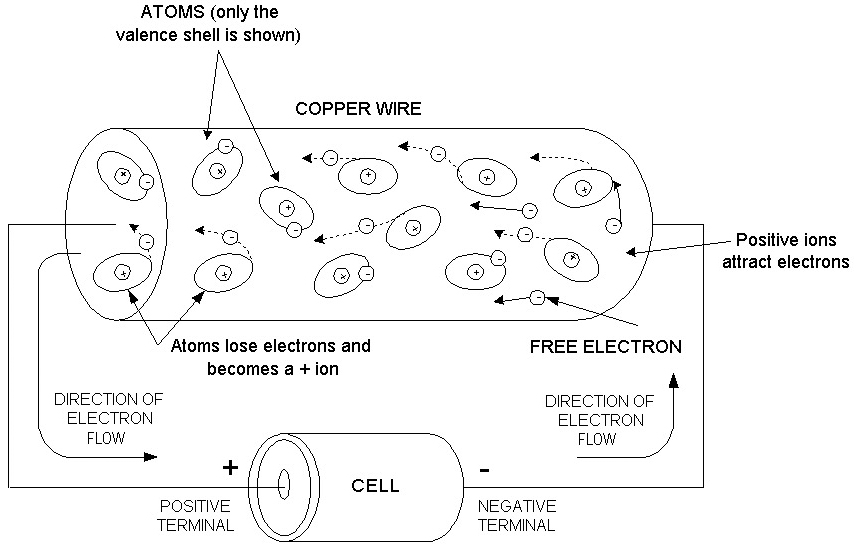
Which way does the current really flow?
Wednesday: Sunprinting
See this week’s handout (at the top of this page) for instructions. Note that you can either rinse the paper under a faucet, or soak it in a tub/pan/etc. Whatever works best for you! If you have lemon juice on hand, you can add some to the water.

What’s happening?
The sun print paper is coated with a photo-sensitive chemical which reacts in light. The photo-sensitive paper turns pale blue when exposed to light. Water stops the chemical process and fixes the shadows of the objects on the sun print paper.
Sun print paper is coated on one side with a photo-sensitive chemical called iron (III) hexacyanferrate (III), Fe[Fe(CN)6], also known as Berlin Green.
Berlin Green is blueish-green and is water-soluble. When exposed to ultraviolet light (UV), a chemical reaction takes place where the water-soluble Berlin Green changes into a water-insoluble chemical called iron (III) hexacyanoferrate(II), Fe[Fe4(CN)6]3, also known as Prussian Blue.
When you submerge the paper in water, the water-soluble Berlin Green washes away, and the water-insoluble Prussian Blue remains fixed on the paper. The intensity of the Prussian Blue depends on the amount of time the paper is exposed to the light source and the intensity of the light source. For example, sun print paper doesn’t work nearly as well on a cloudy day as it does on a sunny day. (Curiodyssey)

Want to learn more about photographic processes?
This framed photograph is an ambrotype, one of the earliest photographic processes. It depicts a Civil War soldier and his family. Learn more about African American history through our Juneteenth historical timeline.
Questions?
Get in touch with Liz, PNG Director:
